


‘The creation of Neocartil® Pro was driven by one main goal:
to devise a premium-category joint formula that is not only effective and innovative, but also unique in its appearance and
complexity.
Neocartil® Pro is the uncompromising embodiment of this idea.
All aspects of joint formulas were looked at afresh
and redesigned for maximum
efficacy and safety.’
A science-based, premium-category product for professional and everyday use.
Learn more about the dosage ofNeocartil™ PRO. >
The key to health in the modern world lies in lifestyle.
Who is Neocartil™ PRO recommended for?Learn more >
Designed from a medical perspective for safe and
continuous use.
Learn more >
Because of its extraordinary complexity, it contains the most important known organic substances essential for the functioning of healthy cartilage, all in a single tablet.
Learn more >Ingredients
read detailed information about
the ingredients of Neocartil™ PRO


Original, non-chemically modified type II collagen
UC-II™ contains native, non-chemically modified type II collagen to support joint functions. It is produced in the United States by a patented, low-temperature, non-enzymatic process. In four clinical studies, 40 mg of UC-II® was shown to reduce joint pain, and to improve mobility and joint flexibility. The clinically proven effective dose of UC-II® is just 40 mg a day. 1
What is native collagen? Collagens are the most abundant family of proteins in the extracellular matrix of connective tissues. The structure of native collagen consists of a characteristic triple helix of polypedide chains rich in glycine, proline and hydroxyproline. Based on their supramolecular structure, 26 different types of collagen can be distinguished. In addition to their biomechanical properties, collagens have several other functions which are strongly dependent on the supramolecular triple helix structure: cell differentiation and gene expression, cell adhesion and migration, and regulation of tissue homeostasis.
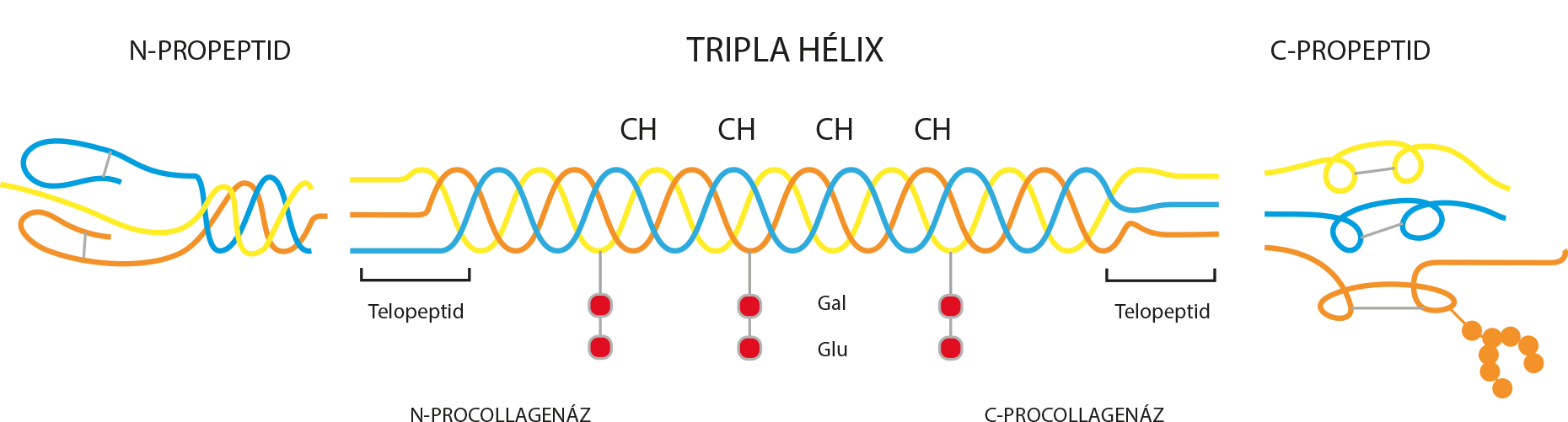
Clinical trials show that UC-II® is more effective than the most commonly used cartilage-strengthening agents glucosamine and chondroitin. 1 UC-ll® is so effective that only a small amount of type II, non-chemically modified collagen is needed to support joint functions. 1

Curcuminoids with up to 10 times higher bio-availability, using PNS technology
Turmeric, a member of the ginger family, has been used in traditional medicine and gastronomy for thousands of years. The main component and most important active ingredient of turmeric is curcumin.
Aurea BioLabs has developed a curcumin with unmatched bio-availability. Cureit is up to 10 times more bio-available than normal curcumin. Both the product and its manufacturing process (PNS technology) are patented, resulting in the only curcumin extract that contains the entire turmeric matrix. The natural matrix of turmeric plays a significant role in increasing the bio-availability of curcuminoids. Protein, dietary fibre, carbohydrates and volatile oils all contribute to the bio-availability of curcuminoids. 1
PNS technology is used to increase the biological efficiency and bio-availability of the product by preserving the natural matrix of the raw material without breaking down the active molecules. Restoring the natural matrix contributes to increasing the stability of the active molecules. 1

In well-controlled clinical trials, the absorption of Cureit was roughly 7 times greater than that of volatile curcumin oil preparations. In comparison with the curcumin-phospholipid formulation, the values were found to be 4.4 and 6.6 times higher. 1
chondroitin - sulfate

The cartilage tissue that covers the joint surfaces is composed of cartilage cells and large amounts of intercellular material produced by the cells. This intercellular matrix determines the mechanical properties of cartilage (friction coefficient, elasticity, resistance). Cartilage cells stop dividing after growth is complete, but continue to produce the large molecules that make up the intercellular material. These compounds are called proteoglycans, which consist of proteins and sugars and have a high water-binding capacity. Glucosamine sulfate and chondroitin sulfate are two very important components of the intercellular matrix of cartilage tissue.
Chondroitin sulfate has the distinctive property of being able to bind water in even larger quantities than glucosamine. With ageing, or in degenerative processes of cartilage tissue, the chondroitin sulfate content of cartilage decreases appreciably, which results in a deterioration of capacity to bind water, leading to a loss of elasticity. This process can then lead to the development of micro-injuries under stress, which can trigger further degradation processes in the cartilage tissue.
The results of experiments have proven that orally administered chondroitin sulfate is safe and is absorbed well.

Osteol™ was developed by Nexira Health of Switzerland from bioactive milk proteins.
This innovative, scientifically supported product is designed to improve the efficacy of the glucosamine + chondroitin mixtures most commonly used in joint preparations and consequently to open up further horizons in joint function and cartilage preservation. 1
Scientific research has shown that Osteol™ may enhance cartilage cell preservation. Furthermore, while maintaining efficacy, the amounts of conventional active ingredients such as glucosamine and chondroitin can be significantly reduced. These claims are based on the results of numerous in vitro and in vivo studies. 1
1,2
Preserving chondrocytes 1
In a clinical trial carried out by Nexira Health, the addition of Osteol™ increased effectiveness from 25% to 95%compared to the usual D-Glucosamine
+ chondroitin combination.
In vitro test: Evaluating the effect of Osteol™ by testing the survival of cartilage cells exposed to stress
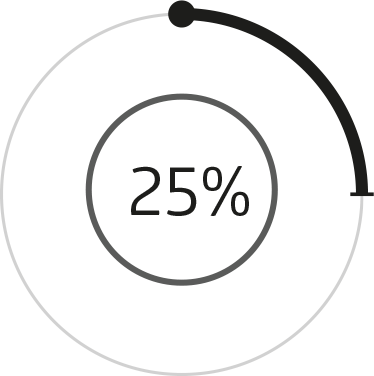
D-Glucosamine
+ chondroitin
Nearly 95%
cartilage cell protection
thanks to Osteol™1
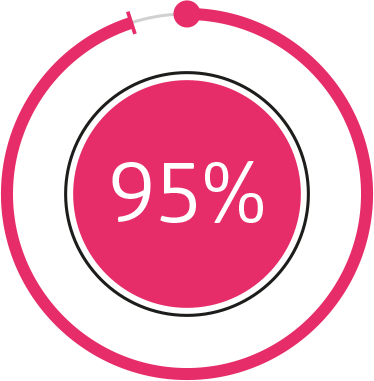
D-Glucosamine
+ chondroitin
+ Osteol™
Thanks to Osteol™, nearly 95% of cartilage cells exposed to stress escape apoptosis compared to the combination of D-Glucosamine + chondroitin with the same concentrations of the active ingredients (1.01 g/ml), where this value was only 25%.
These results, confirmed by other tests
conducted on similar models, prove the exceptional properties of Osteol™. 1
Active dose reduction 2
In another clinical trial conducted by Nexira Health, thanks to Osteol™, D-glucosamine
concentrations could be radically reduced without diminishing the effect.
In vitro test: The relationship between Osteol™ and D-glucosamine, by studying the survival of cartilage cells exposed to stress
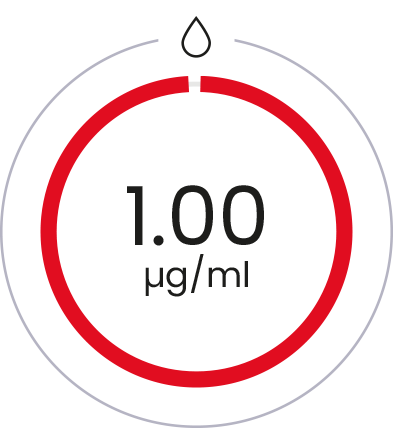
D-glucosamine
alone
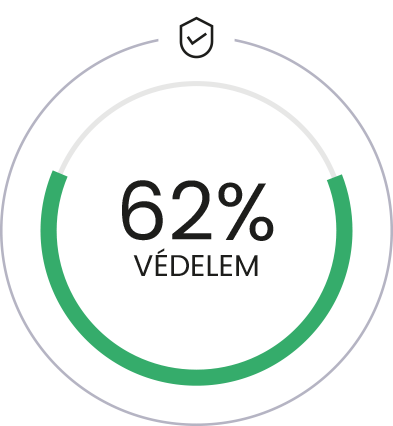
Same effectiveness
with 50 times lower doses
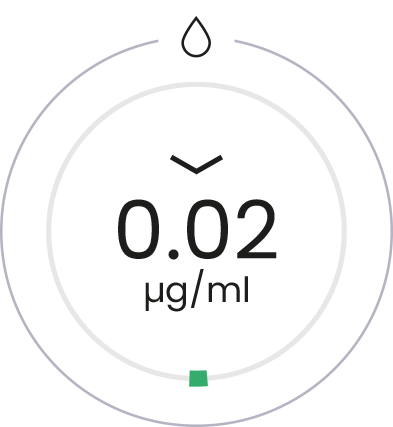
D-glucosamine
+ Osteol™
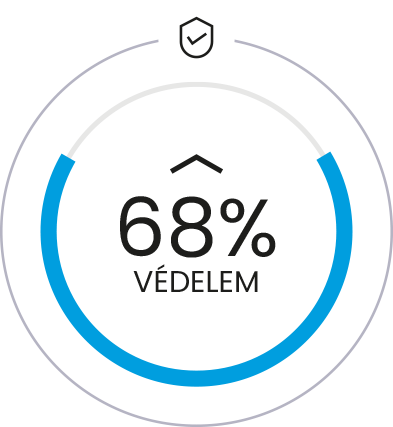
Scientific work has shown that the synergistic effect of Osteol™ correlates
with the ratio of Osteol™ to D-glucosamine. 1

GlucosaGreen® is a plant-derived glucosamine
speciality produced by TSI Group LTD, one of the world’s
largest producers of glucosamine.
GlucosaGreen® is the world’s first commercially available glucosamine, produced by a revolutionary, patented, direct biofermentation process. 1 The main advantage of GlucosaGreen® is that, thanks to the innovative production technology, glucosamine of the same high quality as shellfish glucosamine can be produced without causing serious damage to the environment. 1
Instead of using shellfish as a raw material, the starting material of GlucosaGreen® is ordinary glucose. Glucose comes from a readily available plant source, maize. After glucose is extracted, a biofermentation process takes place and the technology converts glucose into glucosamine very efficiently.
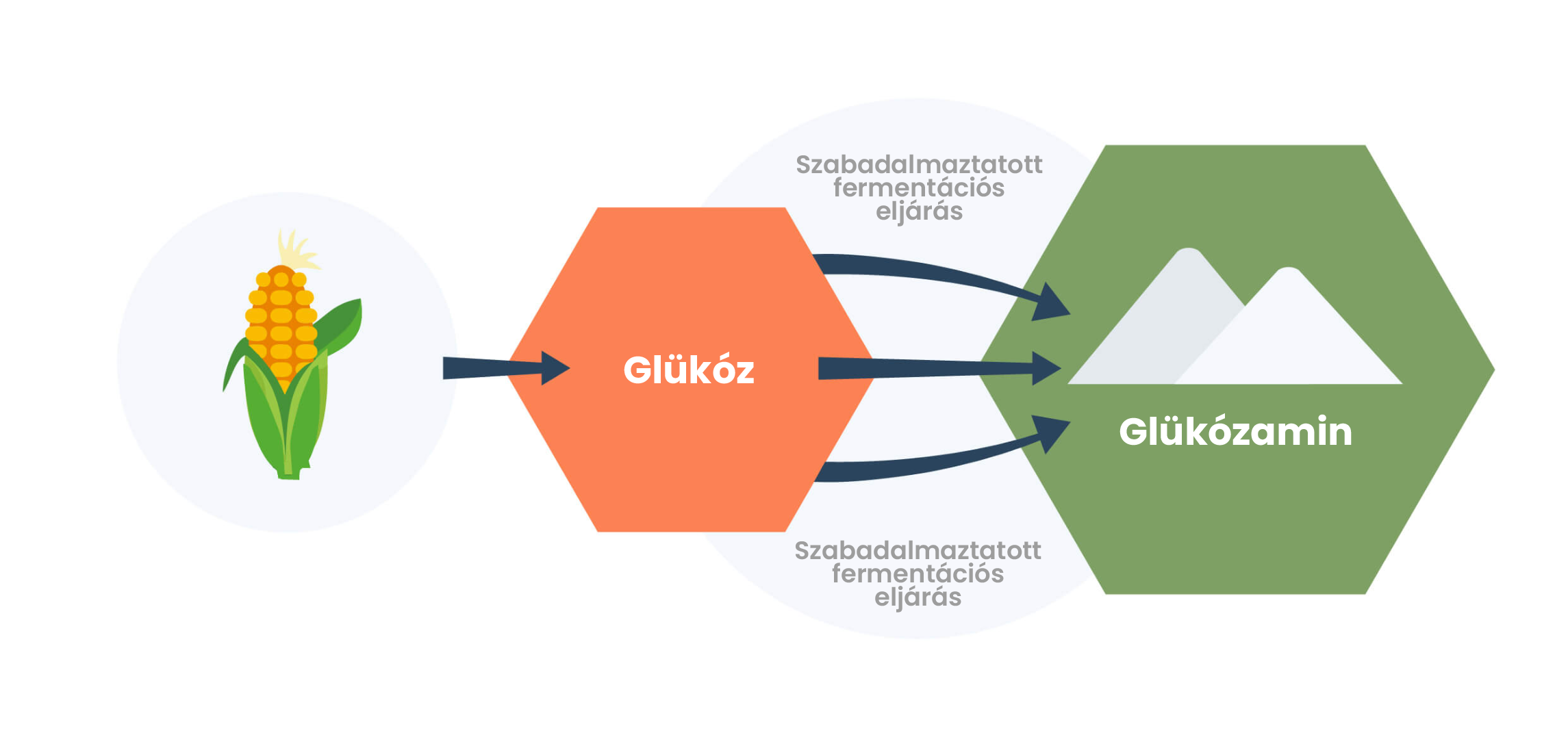
The process eliminates possible allergic reactions arising from animal-based raw materials (such as shellfish) and significantly reduces the ecological footprint of production
1,2
Vitamin C
Apart from its many important physiological roles, vitamin C regulates the synthesis of collagen. Its role is not only to synthesise collagen, but also to maintain the ageing collagen network in the human body. Based on the findings of numerous clinical studies, it has been proved that vitamin C contributes to the normal formation of collagen and thus to maintaining the normal condition and functioning of cartilage. EFSA
hyaluronic acid
Hyaluronic acid (hyalos means glass) forms a viscous, transparent solution and is one of the main components of synovial and vitreous fluids. It is also found in the extracellular matrix of cartilage and tendons, contributing to the flexibility of these tissues. Hyaluronic acid is widely used in degenerative orthopaedic conditions, in the form of both tablets and injections.
MSM
MSM is a sulfur carrier that occurs in large quantities in the human body. MSM is an essential component of the cell membrane, and is present in immune system proteins, connective and epithelial tissues, as well as supportive tissues, and thus in cartilage tissue. MSM is a widely accepted and used ingredient in joint preparations.
manganese
Manganese is a mineral involved in metabolic processes. The human body contains 10-40 mg of manganese and the daily requirement is 1 mg. General symptoms of manganese mineral deficiency: knee pain, dizziness, hearing impairment, poor sense of balance. An antioxidant metal, it is an important component of the superoxide dismutase enzyme system.

Neocartil Pro™ is a registered trademark of SynergoLAB.
Copyright © 2022 SynergoLAB Inc. All rights reserved.